Company
A Regulatory partner specialised in providing top-notch Regulatory affairs and consultancy services to help businesses navigate through the intricate Regulatory procedures.
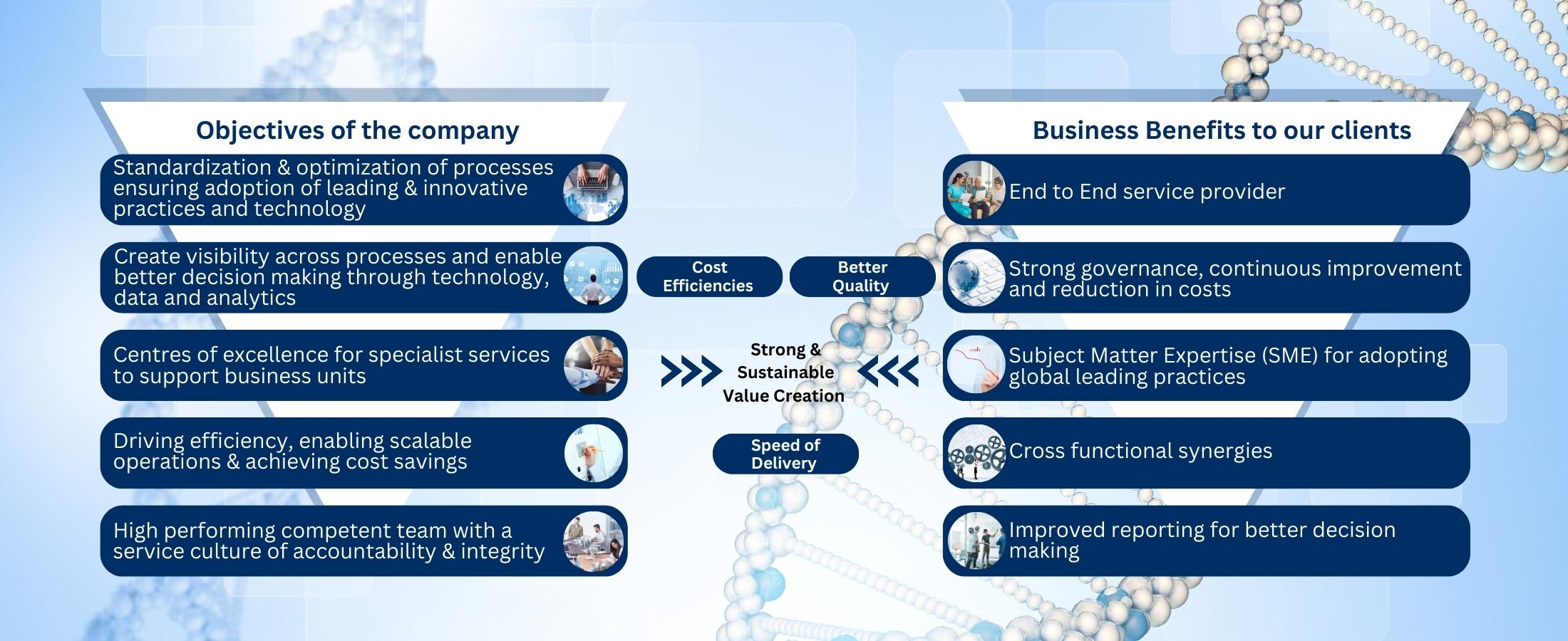
Our primary objective is to facilitate prompt approval, helping clients achieve their goal of launching their products successfully and on time.


At Intelur Pharma, our mission is to be a trusted partner in the pharmaceutical and Bio Pharmaceutical industry, providing comprehensive and cutting-edge Regulatory Services.

Our vision is to be a global leader in pharmaceutical and Bio pharma Regulatory Services, recognized for our excellence, innovation and client-centric approach.

Expertise: We are driven by our passion for knowledge and excellence. Our team of regulatory experts brings extensive experience and in-depth understanding of global regulatory frameworks.
Client Focus: Our clients are at the heart of everything we do. We prioritize their needs, goals and objectives and tailor our services to meet their unique requirements. We strive to build long-term partnerships based on trust, collaboration and mutual success.
Integrity: We uphold the highest standards of integrity, ethics and professionalism in all our interactions. We are committed to transparency, honesty and accountability. Our clients can trust us to handle their sensitive information with utmost confidentiality and safeguard their interests throughout the Regulatory process.
Business benefits we drive
Our Founder
- 32+ years’ experience in Regulatory Affairs.
- Suresh is the driving force behind building the pharma regulator and technical service company globally.
- Formerly, AVP -National Regulatory Affairs at Biocon/ Biologics for 22+ years and worked as Head NRA at Arcolab, a Strides Enterprise for 17 months and prior to Biocon he worked in various pharmaceutical industries for a decade.
- Hands on understanding for Regulatory Requirements of API & Formulation. Numerous successful audits in account from National and International Regulatory bodies & customers.
- Actively involved in various Indian Pharma and Biopharma Associations to identifying critical areas that requires immediate interventions by the relevant Govt. departments to streamline regulatory ecosystem. Received several amendment in D & C act and many Govt. notifications.
- Worked with pharma industries and regulators during drafting guidelines like Guidelines on Similar biologics 2016.
- Experience in NPPA, DBT, IBSC, RCGM and Lego-Regulatory Work strategies for New Drugs, Biosimilars, rDNA, Medical device, during companies’ Merger and De-merger, products under Emergency Use Authorizations (EUA).

Dr. Suresh.S
Our Identity


